5 Things You Didn’t Know About FDA Data-Driven Software Validation
Smart Data Collective
AUGUST 22, 2021
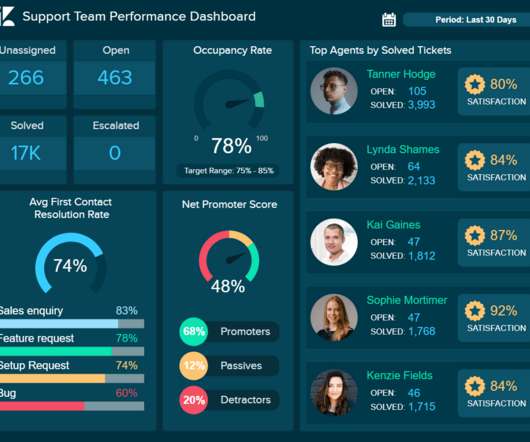
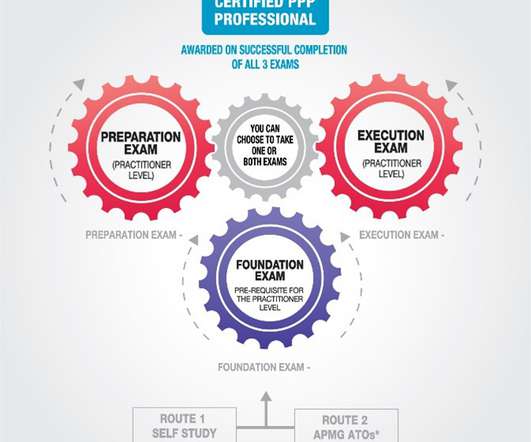
Popularly referred to as computer system validation, FDA software validation is when an organization demonstrates that its software does what it was designed to do. Here’s a quick rundown of the 4Q Lifecycle Model: DQ (Design Qualification): The vendor usually handles this part (if you procured your software from a third-party vendor).











Let's personalize your content